- Teacher: Admin User
faithchepngetich.gnomio.com
-
Welcome to your new Gnomio site
Now, you are in control!
Moodle is an open-source Learning Management System (LMS) that provides educators with the tools and features to create and manage online courses. It allows educators to organize course materials, create quizzes and assignments, host discussion forums, and track student progress. Moodle is highly flexible and can be customized to meet the specific needs of different institutions and learning environments.
Moodle supports both synchronous and asynchronous learning environments, enabling educators to host live webinars, video conferences, and chat sessions, as well as providing a variety of tools that support self-paced learning, including videos, interactive quizzes, and discussion forums. The platform also integrates with other tools and systems, such as Google Apps and plagiarism detection software, to provide a seamless learning experience.
Moodle is widely used in educational institutions, including universities, K-12 schools, and corporate training programs. It is well-suited to online and blended learning environments and distance education programs. Additionally, Moodle's accessibility features make it a popular choice for learners with disabilities, ensuring that courses are inclusive and accessible to all learners.
The Moodle community is an active group of users, developers, and educators who contribute to the platform's development and improvement. The community provides support, resources, and documentation for users, as well as a forum for sharing ideas and best practices. Moodle releases regular updates and improvements, ensuring that the platform remains up-to-date with the latest technologies and best practices.
Links of interest:
(You can edit or remove this text)
Available courses
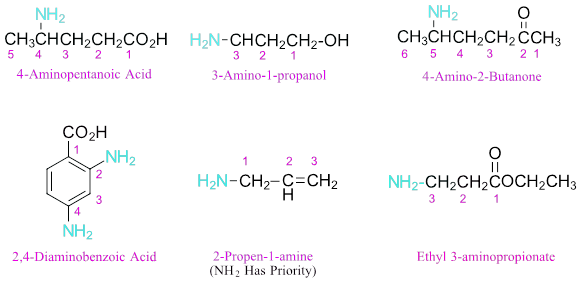
Organic chemistry is the study of carbon-based compounds, focusing on their structure, properties, reactions, and synthesis. It’s foundational for careers in medicine, biology, chemistry, and environmental science.
Overview of Organic Chemistry
Organic chemistry explores the vast world of carbon-containing molecules, which form the basis of life and many synthetic materials. The course typically spans two semesters:
Organic Chemistry I
- Structure and Bonding
- Hybridization, resonance, formal charges
- Lewis structures and molecular geometry
- Acid-Base Chemistry
- pKa values, conjugate acids/bases, equilibrium
- Functional Groups
- Alkanes, alkenes, alkynes, alcohols, ethers, amines, halides
- Stereochemistry
- Chirality, enantiomers, diastereomers, optical activity
- Reaction Mechanisms
- Substitution (SN1, SN2), elimination (E1, E2)
- Reactions of Alkenes and Alkynes
- Addition reactions, Markovnikov vs. anti-Markovnikov
Organic Chemistry II
- Carbonyl Chemistry
- Aldehydes, ketones, carboxylic acids, esters, amides
- Nucleophilic Addition and Substitution
- Mechanisms and reactivity patterns
- Enols and Enolates
- Keto-enol tautomerism, aldol reactions
- Aromatic Compounds
- Electrophilic aromatic substitution, directing effects
- Spectroscopy and Analysis
- IR, NMR, and mass spectrometry for structure determination
- Multistep Synthesis
- Designing synthetic pathways using known reactions
Skills Developed
- Predicting and explaining chemical reactivity
- Drawing mechanisms and understanding electron flow
- Applying spectroscopy to identify unknown compounds
- Designing synthetic routes for complex molecules
Helpful Resources
- Khan Academy’s Organic Chemistry Course offers free video tutorials and practice exercises
- Teacher: Admin User
-
Definition: Organic chemistry is the study of carbon-based compounds, especially those containing carbon–hydrogen (C–H) bonds.
-
Scope: It covers everything from simple molecules like methane to complex biomolecules like DNA and proteins.
-
- Teacher: Admin User
Physical chemistry is a branch of chemistry that explores how matter behaves on a molecular and atomic level and how chemical reactions occur. It blends principles from physics and chemistry to understand the physical properties of substances and the mechanisms behind chemical processes.
Key Concepts in Physical Chemistry
-
Thermodynamics: Studies energy changes, heat, and work in chemical systems.
-
Kinetics: Examines the speed of chemical reactions and the factors that influence them.
-
Quantum Chemistry: Uses quantum mechanics to explain the behavior of atoms and molecules.
-
Spectroscopy: Investigates how matter interacts with electromagnetic radiation.
-
Statistical Mechanics: Connects microscopic particle behavior to macroscopic properties like temperature
Purpose and Scope
-
Understand the fundamental laws governing chemical reactions.
-
Predict how substances will behave under different conditions.
-
Develop new materials and technologies by manipulating molecular interactions.
As Britannica puts it, physical chemistry is “concerned with interactions and transformations of materials,” and it seeks to explain the quantitative aspects of chemical phenomena using physics-based models. You can also explore a deeper overview
- Teacher: Admin User
Laboratory practice is a broad term, but most often refers to Good Laboratory Practice (GLP), a quality system for non-clinical laboratory studies that ensures the quality, validity, reliability, and reproducibility of the data produced. GLP provides a framework for the organization, planning, performance, monitoring, reporting, and archiving of studies related to the health and environmental safety of products like pharmaceuticals, chemicals, and food additives
- Teacher: Admin User
Inorganic chemistry is the branch of chemistry that studies the properties, reactions, and synthesis of compounds that do not contain carbon-hydrogen bonds, encompassing all elements and compounds on the periodic table except for those typically classified as organic.
- Teacher: Admin User

Inorganic Chemistry is a branch of chemistry that deals with the properties and behavior of inorganic compounds. This course provides a comprehensive overview of the fundamental concepts and principles of inorganic chemistry, including:
-
Chemical Bonding: Explore the nature of ionic, covalent, and metallic bonds, and understand molecular geometry and hybridization.
-
Coordination Chemistry: Study coordination compounds, their structures, bonding theories, and the role of ligands.
-
Main Group Elements: Examine the chemistry of the main group elements, including their reactivity, trends in the periodic table, and applications.
-
Transition Metals: Investigate the unique properties of transition metals, including their electronic configurations, oxidation states, and their role in catalysis.
-
Bioinorganic Chemistry: Understand the significance of metals in biological systems, including metalloproteins and metalloenzymes.
-
Solid State Chemistry: Explore the structure, properties, and synthesis of inorganic solids, including crystallography and defects.
Learning Outcomes:
By the end of this course, students will be able to:
- Explain the fundamental principles of inorganic chemistry and apply them to solve problems.
- Analyze the structure and bonding of inorganic compounds using various theories.
- Demonstrate knowledge of the role of inorganic substances in biological and industrial processes.
- Conduct laboratory experiments to synthesize and characterize inorganic compounds.
- Teacher: Admin User
